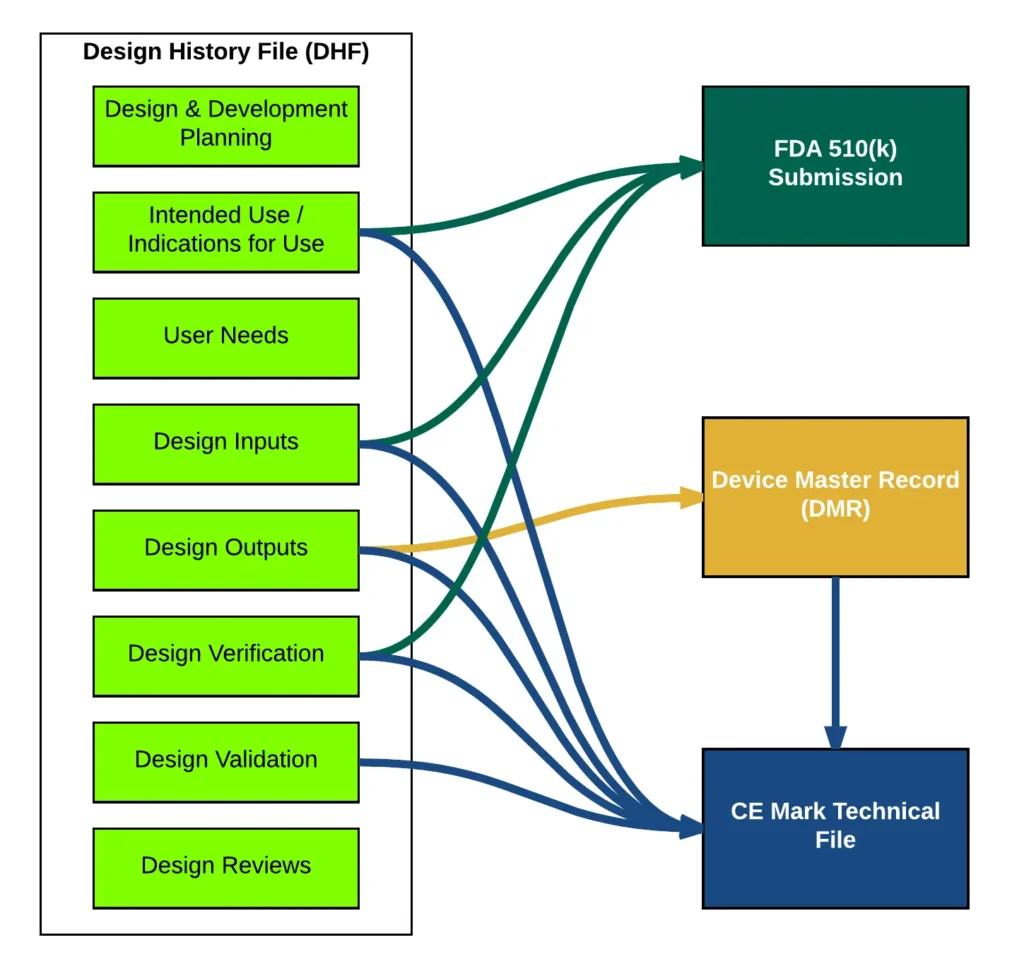
Ensuring medical devices meet safety standards is crucial for protecting patients and building trust. Preparing technical files accurately is essential to comply with strict regulatory requirements in the healthcare industry. Professional help simplifies navigating complexities, ensuring compliance while saving time and reducing potential errors. Rigorous testing guarantees that devices perform properly and meet the highest standards for market approval.
An experienced FDA 510k consultant provides valuable guidance to streamline submissions and achieve regulatory clearance. Their expertise ensures your device meets standards while addressing every aspect of the submission process. Here, we focus on the benefits of seeking expert assistance for successful FDA 510k submissions. Continue reading to discover how professional support ensures compliance and boosts your medical equipment’s success.
Compile Detailed and Accurate Data
Accurate and detailed data forms the backbone of successful technical files for FDA submissions. Include comprehensive descriptions of your device, intended use, and performance specifications. Provide supporting evidence such as test results, risk assessments, and engineering reports in an organized manner. Reliable advice from legal consultants ensures your documentation is error-free and meets all compliance requirements. Well-prepared documentation reflects your commitment to quality and compliance in medical equipment development.
Include Robust Safety and Performance Testing Results
Demonstrating device safety and performance is critical to the FDA 510(k) submission process. Conduct rigorous preclinical and clinical tests to validate your tool’s functionality and reliability. These findings clearly show how your device meets or exceeds applicable benchmarks. Regulatory consultants with proven expertise provide valuable support in ensuring your testing protocols meet FDA recommendations. Transparent and thorough testing results build trust in the safety and effectiveness of your equipment submission.
Maintain a Clear and Organized Structure
A well-structured technical file makes reviewing and evaluating submissions easier for the FDA. Divide your documentation into logical sections, such as device description, test results, and labeling information accurately. Use consistent formatting and organized tables to ensure your information is concise and accessible to reviewers. Legal advisors with industry expertise can recommend improvements to simplify documentation and enhance clarity. An organized structure demonstrates professionalism and makes your submission more reviewer-friendly.
Keep a Focus on Substantial Equivalence
Proving substantial equivalence to a legally marketed predicate device is central to FDA 510(k) submissions. Provide a detailed comparison highlighting similarities and differences between your equipment and the predicate device thoughtfully. Use precise language to explain why your tool is equally safe and effective as the predicate clearly. Professional guidance ensures your comparison includes compelling charts, tables, or visual aids for optimal impact. Establishing substantial equivalence builds confidence in your device’s compatibility with existing market standards.
Collaborate with Regulatory Experts
Working with seasoned legal professionals ensures your technical files meet the highest compliance standards. Their expertise can assist in presenting technical data effectively and addressing potential queries. Regulatory specialists help streamline the submission process by identifying gaps and improving documentation. Reliable advice from experienced consultants helps you stay updated with regulatory changes and prevent unexpected issues. Collaboration with experts provides valuable insights and boosts your confidence throughout submission.
Ensure Timely Updates to Technical Documentation
Maintaining current and updated technical files is crucial for successful FDA 510(k) submissions every time. Review and revise your documentation to reflect any equipment design or use changes. Consistent updates ensure your technical files align with evolving regulatory standards. Trusted consultants with expertise in regulatory compliance can assist in identifying necessary updates. Keeping technical files up-to-date reinforces your commitment to transparency and compliance during the FDA submission process.
Navigating FDA regulations can feel overwhelming, but expert guidance simplifies the process significantly. A trusted FDA 510k consultant ensures your submissions are accurate, efficient, and aligned with regulatory expectations. Their support helps avoid costly delays, keeping your medical tools journey on track. Reliable agencies provide personalized insights tailoring strategies to meet your product’s unique compliance needs. Reach out today and empower your business with expert assistance for seamless regulatory approval success.
Also Read-Why Working with a Reliable Pneumatic Ball Valve Supplier Matters