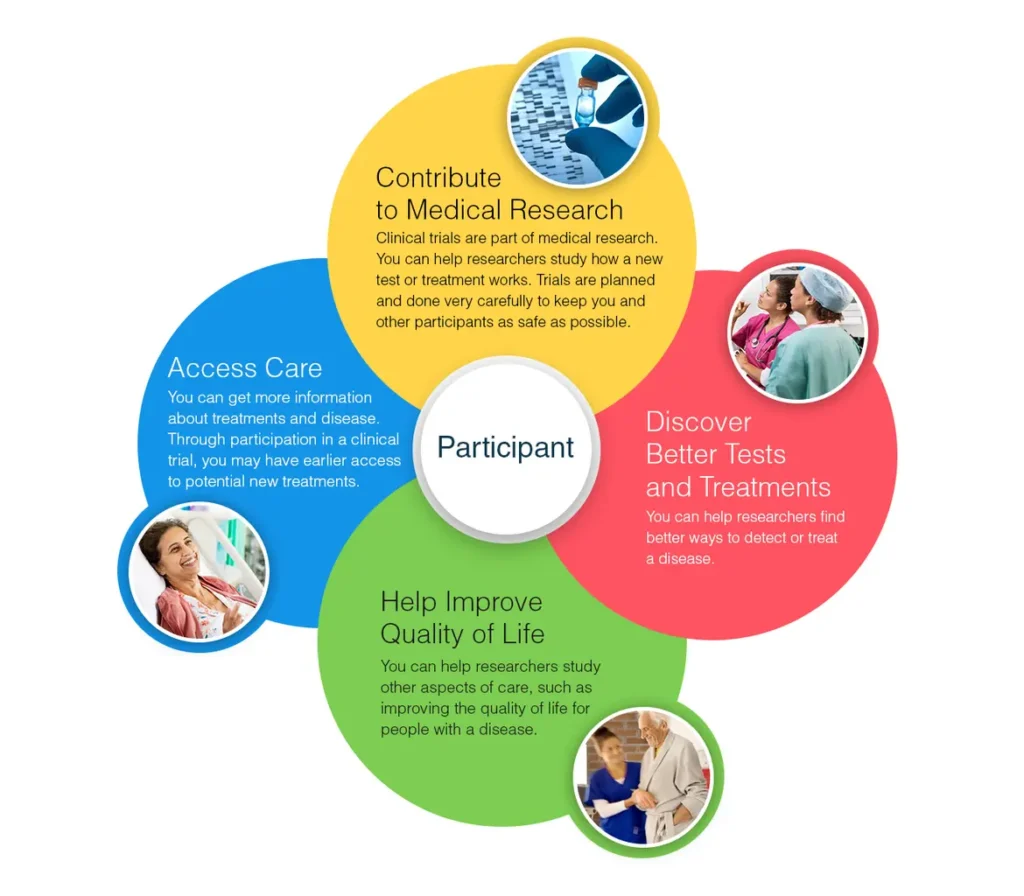
Key Takeaways
- Understanding the clinical trial process can empower potential participants.
- Participation in clinical trials offers insights into cutting-edge medical research.
- Clear communication and careful preparation benefit both researchers and participants.
The Clinical Trial Process Overview
Clinical trials are pivotal in advancing medical research and improving patient care. These carefully structured studies are essential for evaluating new treatments, drugs, and medical devices and determining their efficacy and safety. When individuals participate in these trials, they not only stand a chance to access groundbreaking therapies but also contribute significantly to the progress of science and medicine. Whether it’s understanding the intricacies of clinical trials for lung cancer or exploring other conditions, having a well-rounded comprehension of these trials is crucial for prospective participants.
There are various stages to the clinical trial process, each focusing on a different research objective. Phase I trials prioritize safety by testing treatments on a small group of people, often determining the ideal dosage range. Phase II shifts focus towards efficacy, ensuring the treatment effectively impacts the condition. During Phase III, researchers compare new interventions with existing standard treatments to validate their benefits. When a trial reaches this phase, the sample size is significantly larger, providing broader data for analysis. Organizations like the FDA are deeply involved in overseeing these phases to maintain rigorous standards for safety and privacy.
Eligibility and RecruitmentEvery clinical research study includes precise eligibility requirements intended to guarantee the reliability of study data and participant safety. These criteria may include age, gender, underlying health conditions, and medical history, which help researchers focus on the study’s specific requirements. Participants meeting these criteria can provide consistent and comparable data, which is crucial for drawing reliable conclusions about the treatment’s efficacy and safety.
Recruiting participants is a strategic process involving outreach and education to explain participation and the potential contributions to science and individual health. A diverse pool of participants enhances the robustness of a clinical trial, promoting findings applicable to various demographics across different populations. Increasingly, efforts are being made to ensure these trials are inclusive, breaking barriers related to ethnicity, age, and socioeconomic status, ultimately fostering a comprehensive understanding of the treatment across different groups.
Risks and Benefits of Participating
Embarking on participation in clinical trials involves weighing potential risks against potential benefits. While every trial is carefully monitored to minimize risks, adverse reactions or ineffective treatments remain possibilities that participants must consider. These include unexpected side effects, which researchers continuously assess to ensure participant safety. Nonetheless, the opportunity to advance new therapies and receive treatments before they are widely available can benefit participants significantly.
The potential impact a successful trial can have on public health further underscores the importance of participation. For instance, successful trial outcomes have led to the development of life-saving drugs and improved therapeutic approaches that have transformed healthcare paradigms. These advancements demonstrate that, despite inherent risks, clinical trials are a cornerstone of medical innovation, offering hope and better health outcomes.
Informed Consent Process
Informed consent is a fundamental ethical component of clinical trials, ensuring participants fully understand the nature of the trial before they begin. The process involves comprehensive dialogue where researchers convey all pertinent information, including trial objectives, procedures involved, potential risks, and benefits. This transparency is crucial for empowering participants and helping them make informed, autonomous decisions about joining the trial.
Prospective participants are encouraged to engage with researchers, asking questions and seeking clarifications to grasp their participation’s potential implications fully. This communication phase improves the overall integrity and efficacy of the clinical trial by fostering a collaborative partnership and trust between participants and researchers.
Preparing for a Clinical Trial
Thorough preparation is vital for anyone considering participation in a clinical trial. To meet the trial’s eligibility requirements, participants must collect comprehensive medical records and undergo necessary health assessments. These steps confirm their suitability and establish a health baseline for researchers to reference as the study progresses.
Open communication with the research team and personal healthcare providers can significantly enhance the trial experience. Such proactive engagement allows individuals to voice concerns and understand how participating might affect other aspects of their healthcare. This collaboration is critical in addressing uncertainties and fostering a supportive environment throughout the trial.
Life During a Clinical Trial
Daily experiences in a clinical trial can differ significantly depending on the type of study. Participants may be required to attend regular check-ups, undergo frequent assessments, and complete various tasks to help researchers gather comprehensive data. Adhering to the trial’s protocol ensures that the data collected is valuable and reliable.
Support systems are essential in helping participants navigate the trial process. Healthcare professionals, trial coordinators, and sometimes peer networks provide assistance and reassurance. These support structures play a critical role, offering emotional and practical help, thus enhancing the participant’s experience and overall satisfaction with the trial.
Post-Trial Considerations
Following the conclusion of a clinical trial, participants enter a phase often characterized by follow-up activities. These follow-ups are crucial for monitoring the participant’s health and documenting any delayed reactions to the treatment. Such longitudinal data is invaluable in understanding the long-term efficacy and safety of the interventions studied.
The insights gained from these trials often feed back into medical practice, influencing treatment guidelines and future research directions. The significant contribution of participants to medical developments is highlighted by this ongoing cycle of research and implementation, opening the door for novel treatments that can help larger populations.
Resources and Support Networks
Numerous resources are available to support participants through their clinical trial journey. Comprehensive platforms like ClinicalTrials.gov offer valuable information and network connectivity to assist the trial process. These resources facilitate informed decisions and enhance participant experiences by providing guidance and essential support.
Engaging with these networks can be particularly beneficial. They connect participants with peers and professionals who understand the clinical trial landscape. This community support fosters a sense of belonging and shared purpose, empowering participants to contribute confidently to advancing science and healthcare.
Also Read-How PCB Design and Assembly Drive Technological Innovation